Boyle's Law In Real Life
In life, aught happens randomly or without reason. Even those events that you consider tiny are all fully dependent on the laws of nature. Everything happens for a reason, and according to firm laws, do not allow randomness to dominate the universe .
And then, this article revolves effectually some applications that occur in our lives are thought to be random; nevertheless, they are based on one of the nigh of import physical laws, which is Boyle'southward law(real life applications of Boyle's law) and why is Boyle'due south constabulary of import .
At the stop of the article, at that place will exist a 3D simulation at the physics lab in PraxiLabs to prove Boyle's police force.
Try 3D Virtual Labs Now
Who is Boyle?
Boyle is a pharmacist and physicist and also an inventor. Although he suffered from a serious disease that afflicted his eyes permanently, he overcame his disability and hired some people to write his thoughts.
Boyle became the starting time to put the foundation stone in gases laws and to find a law that reflected the beliefs of gases. He discovered a breakthrough in physics which then became chosen Boyle's law. He was also one of the founders of modern chemistry.
What is strange is that he did not officially attend whatsoever academy. Boyle was so rich that he did all his research at his own expense. Despite his achievements in physics, his favorite subject was chemical science.
How did Boyle discover the first law of gases – (Boyle'due south law)?
In 1654, the German physicist Otto von Gerecke invented the beginning evacuated tube. When Boyle learned virtually it, he discussed this invention with his friend Robert Hook.
Hook worked on improving this vacuum tube, then Boyle and Claw began to discover air and vacuum properties using this tube.
While Boyle and Hook were doing their experiments, they discovered the greatest discovery of their lives, now called Boyle's law. They inverse the pressure level on a fixed weight of air using mercury. Boyle discovered that the greater the force per unit area on the air, the smaller the volume.
What is Boyle's law and what is its significance? (significance of Boyle's law)
When the pressure changes on a sure corporeality of gas, its size is inversely proportional to the pressure, provided that the temperature is constant.
The law is described by the mathematical equation PV = K. Information technology has get a basic police force in chemistry.
The Importance of Boyle'southward police force
The importance of Boyle'southward law lies in being the first law to describe the behavior of gases.
Information technology explained that the gases spread in the medium, that is, the volume increases if the pressure is decreased and vice versa (the particles are displaced from each other and movement easily) if the gas is compressed, causing the book to shrink.
Create a FREE Virtual Labs Business relationship At present!
Different Boyle's police applications in existent life
1- Spray pigment Boyle's police
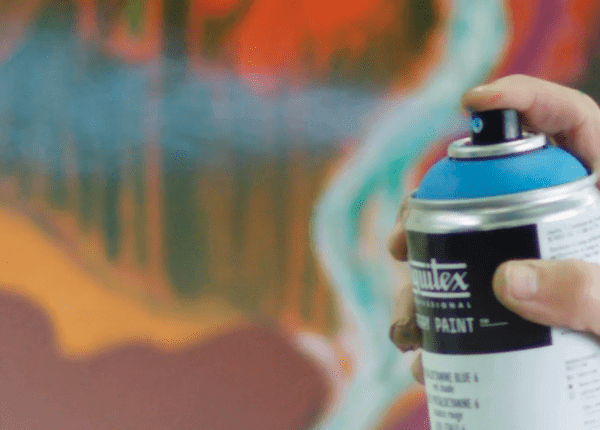
Y'all may have wondered how Boyle's law is applied in aerosols?
Spray paint or aerosol spray is consider 1 of applications of Boyle's police, as information technology is by and large based on Boyle'due south law, where the paint container contains 2 substances, one of them is the paint fabric itself, and the other is a compressed gas in a liquid state in the container.
Although the liquefied gas boiling bespeak is less than room temperature, it does not actually eddy in the container and does not turn into gas considering the container is best sealed.
As soon equally you press the sprayer and the gas starts to get out of the container, the boiling country starts, the liquefied gas expands and turns into gas, and the gas presses the paint inside the container. The pigment textile is pushed up to go out of the sprayer nozzle with gas escaping from the container.
ii- Soda bottle (Soda can Boyle's law)
Soda bottles or cans are consider a practical application of Boyle's law ,as all of us apply Boyle's Police force but unintentionally. Note that when you open up the bottle of soda rapidly, the gas rushes from everywhere in the course of foam, causing a mess. So What is the cause of this mess?
 This mess occurs because the soda is pumped into the soda canteen past passing the water on carbon dioxide. When you open up the bottle, you are really reducing the pressure on the gas, and the volume of the gas expands.
This mess occurs because the soda is pumped into the soda canteen past passing the water on carbon dioxide. When you open up the bottle, you are really reducing the pressure on the gas, and the volume of the gas expands.
If yous remove the cap chop-chop, the gas pushes out of the bottle. Therefore, yous should open the cap slowly and carefully until the gas comes out quietly.
Nosotros are facing another phenomenon in the cases of soda bottles, which is the effervescence of soda if the bottle exposed to shaking. Then what happens in this instance?
In this case, when the cap begins to open, the gas tries to escape from the bottle. But, because of being mixed with the liquid, the gas brings the fluid with it and they are pushed out together, turning into cream and causing a mess.
Go Started Praxilabs For Gratis
3 – Diving into deep water

Every skillful diver knows that, after diving in deep water, divers have to return to the top very slowly. Our bodies are built and designed to alive in natural pressure. Low and increased pressure crusade many problems. Here is the scientific reason behind the slow ascent.
As the diver moves down to the lesser of the water, the force per unit area increases. Increasing pressure level leads to a decrease in volume, and the diver'south blood begins to absorb the nitrogen gas. The opposite happens when the diver starts to ascent over again, and the nitrogen gas molecules begin to aggrandize and return to its book.
If the diver makes a slow ascension, the nitrogen gas molecules expand and return to normal without problems, just if information technology rises apace, the diver'southward blood turns into foam and the same mess that occurs in the soda bottles causes the diver to bend and feel stiff hurting.
In the worst case, this sudden drop in trunk pressure level tin can instantly terminate the diver's life.
Lookout the following video, for a better caption of changing of the volume as the pressure level changes below the sea level.
An experiment to prove Boyle'southward constabulary at the physics lab in PraxiLabs.
Later on recognizing Boyle, his first police force of gases and also applications of Boyle'south law , nosotros thanks for your reading of the article to the end, and we invite you to endeavour the virtual lab and do a 3D simulation to show Boyle's police via PraxiLabs.
Steps to admission the experiment:
- Create a costless account
- Click on My account
- Press 3D simulations
- Click on Physics
- Select Boyle'south Law
Finally, we wish you a pleasant experience with our lab. Feel complimentary to tell united states how your feel was with PraxiLabs.
Join Us At present for FREE
Boyle's Law In Real Life,
Source: https://blog.praxilabs.com/2019/05/16/applications-of-boyles-law-en/
Posted by: rathcatill.blogspot.com


0 Response to "Boyle's Law In Real Life"
Post a Comment